Quality Test Management SolutionWaters NuGenesis LMS
Waters® NuGenesis® LMS (hereinafter referred to as LMS) is a system that centralizes all data related to quality testing. Test instrument data and raw data are managed using the Scientific Data Management System (hereinafter referred to as SDMS), a scientific information data management system, and laboratory notes are created using the Electric Laboratory Notebook (hereinafter referred to as ELN), ensuring data integrity throughout the data lifecycle.
As a Waters Informatics Strategic Partner, B-EN-G will support the introduction of NuGenesis, including license sales, server installation, system management settings, analysis and test record template creation, device connection settings, and the construction of integration with other systems. Click here for Waters' product page.
Special Project
Dialogue Series "Creating the Future of Healthcare Together" Part 1: Waters Japan
"Transforming Labs for the Biopharma Era: Responding to Technological Trends and Tighter Regulations" (Published September 2025)
The article is Here
Overview
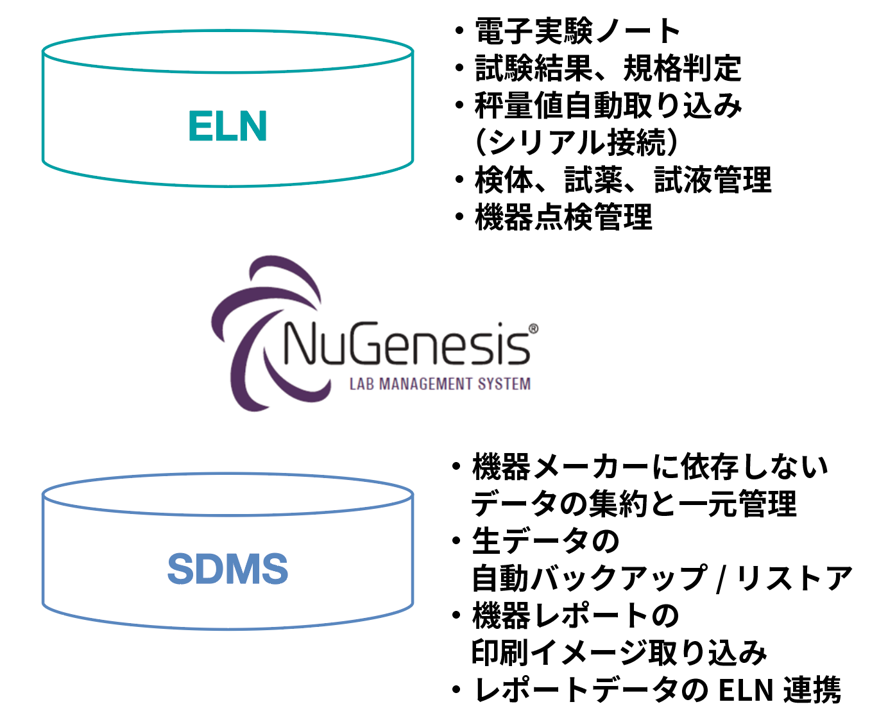
The LMS consists of two modules, Scientific Information Data Management System (SDMS) and ELN.
SDMS automatically backs up raw data files, imports and centrally manages report print images from analytical instruments, regardless of manufacturer or type.
ELNs can record instrument data, final calculations, etc., within electronic lab notebooks, including electronic signatures for review and approval, all in one system.
The biggest feature of NuGenesis LMS is that you can freely define test records even if you are not a system expert, and laboratory members can independently maintain and maintain test records.
function
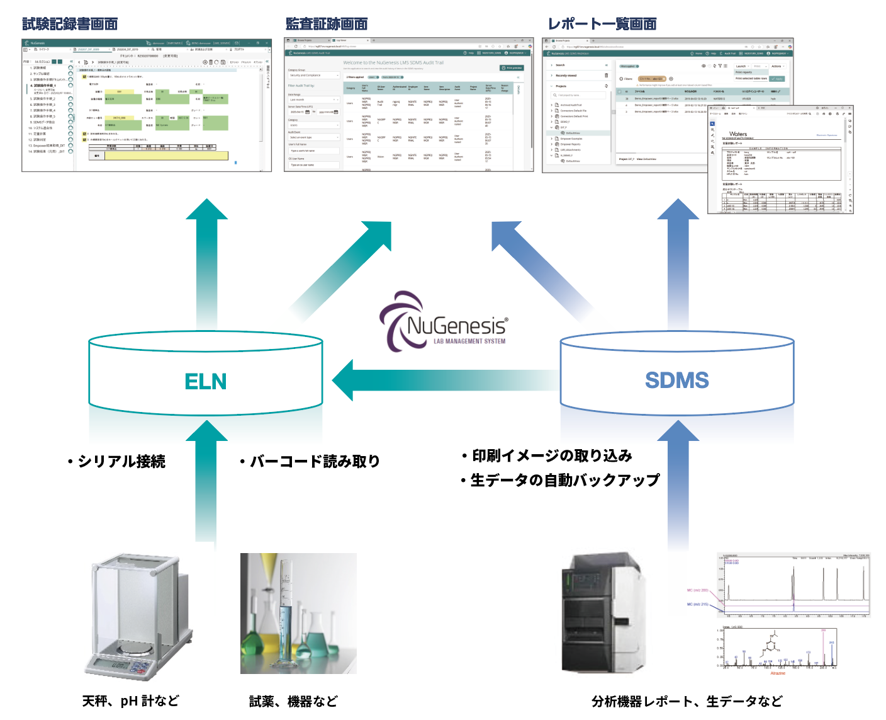
- SDMS
-
- Regardless of manufacturer or type of analytical equipment, it automatically backs up raw data files and imports print images of reports from analytical equipment for centralized management.
- It supports both analysis data as well as PDF, Word, Excel, and data from various applications.
- ELN
-
- Excel and Word can be used directly within the notebook, and data from analytical instruments imported into SDMS can be used flexibly.
- Measurement data can be directly imported from analytical equipment with RS232C serial communication function.
- Sample management, reagent/reagent management, and analyzer management functions are also available.
- With the addition of the stability test option, manage everything from stability test planning to statistical analysis of results.
Example of Data flow when Using SDMS
Benefits
- Regulatory compliance
-
- Data integrity compatible
- Compatible with laws and regulations of each country
- Audit trail (GMP, GQP, GCP, GLP, etc.)
- Electronic signature
- Security measures
- Centralized management of test data and raw data
- Data from various analytical instruments and files such as Word and Excel can be imported into SDMS and managed centrally.
- Digitization of experiment notebooks (paperless)
- A single system can manage not only manual records, equipment data, final calculation result data, etc. generated during analysis work, but also reviews and approval records using the workflow function.
Efficient Implementation with B-EN-G’s data Integrity Templates
At B-EN-G, based on our experience with multiple NuGenesis implementations, we have created components that can be shared, such as function settings, and have prepared them as templates.
When introducing a new system, the "DI Compliance Template" can be used as is or with partial modifications for efficient introduction.
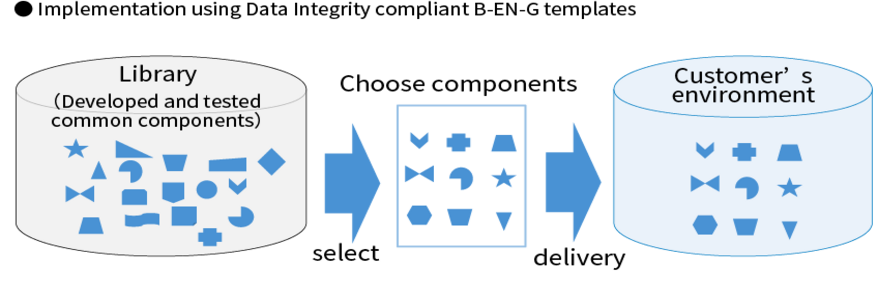
- Advantages of introducing DI compatible templates
-
- Quality assurance
- Short-term introduction
- Improving the efficiency of testing work
- Cost reduction
Download Document
You can download the following materials.
- Data Integrity in the Pharmaceutical Industry and B-EN-G's Quality Test Management Solution ~NuGenesis LMS
An explanation of the data integrity required in the pharmaceutical industry, the role played by the quality test management solution NuGenesis LMS, and B-EN-G system implementation support services such as CSV. - NuGenesis Overview
Describes issues in quality testing operations, the direction of their resolution, and an overview of the quality testing solution "NuGenesis" - NuGenesis Screen Collection
Describes representative screens and explanations for each function in the "Operation Image" chapter of the "NuGenesis Overview" above.

- Download Data Integrity Measures Materials
We have prepared materials that summarize key guides, guidance, and specific solutions related to data integrity.

