Computerized System Validation (CSV) Support Service
In recent years, many companies have been outsourcing all processes, including CSV, to IT companies. However, this is not a desirable situation from the perspective of the separation of manufacturing and quality departments, which is the basic principle of GMP, and validation and qualification.
In addition to the system development and implementation team, B-EN-G has organized a specialized team that provides CSV support services, which includes multiple consultants who have worked in quality assurance at pharmaceutical companies and have numerous experiences in CSV activities and responding to FDA inspections.
B-EN-G CSV method
The execution and reliability of the results of various tests are evaluated from the perspectives of whether they were carried out in an appropriate manner, whether they were performed by the appropriate personnel, and whether this can be confirmed from the records, and pass/fail is determined depending on whether the tests meet the evaluation criteria.
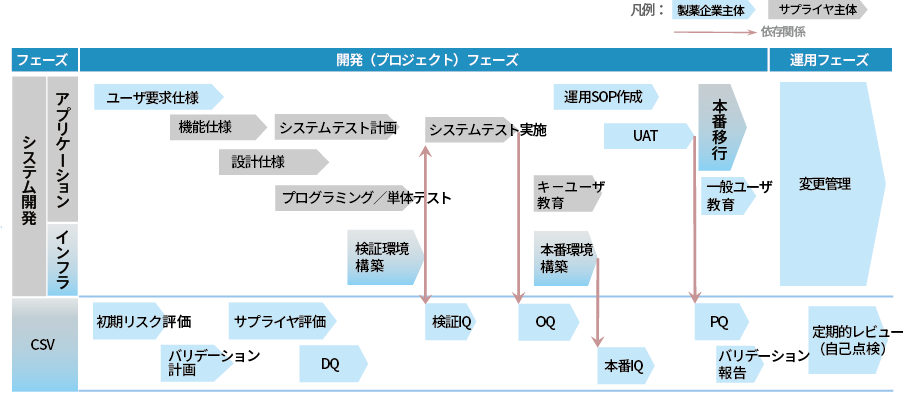
This method is also the same for DQ: evaluation of design-related documents, IQ: evaluation of installation-related documents, OQ: evaluation of function verification-related documents such as system tests, and PQ: evaluation of operation-related documents such as UAT and procedure manuals.
- Separating system development activities led by IT companies from CSV activities led by pharmaceutical companies, each working independently and efficiently
- A verification environment is built at the customer's site early on, and system testing is carried out in the verification environment. The existence of a verification environment makes it easier to manage changes after operation, and minimizes the downtime of the production environment.
- IQ is performed in both production and validation environments to ensure equivalence
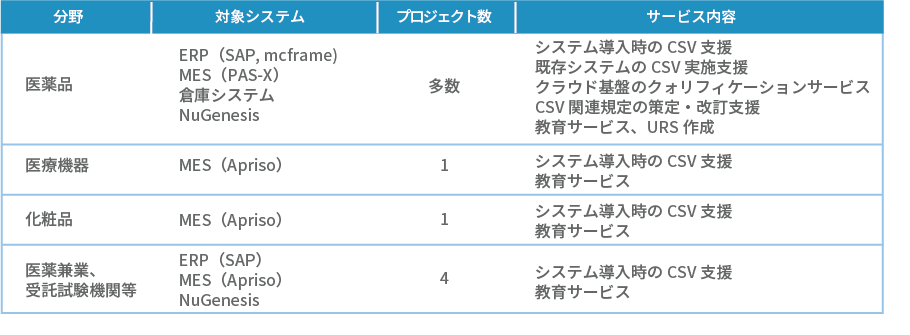
A track record of over 30 companies, mainly pharmaceutical manufacturers
With the advancement of IT technology, there is an increasing tendency for confusion to arise when implementing CSV, but we will support pharmaceutical companies by seeing things from their perspective and ensuring that validation activities are rational and in line with the requirements of regulatory authorities.
Related Solutions
CSV on AWS(Computerized System Validation on Amazon Web Service)
We provide two documents that need to be considered when applying CSV: "AWS CSV Compatibility Reference" and "AWS Supplier Checklist Reference".

