CSV on AWS (Computerized System Validation on Amazon Web Service)
The pharmaceutical and medical device industries today require flexible and rapid responses to changes in the business environment, the creation of new business opportunities using big data, compliance with international standards and country-specific regulations when expanding overseas, and further cost optimization. Amazon Web Services (AWS) is a secure, flexible, low-cost, and high-quality cloud environment that can be rapidly deployed globally.
In January 2016, we created and provided two documents necessary for considering the application of CSV: "AWS Usage Reference for Companies Subject to the Pharmaceutical and Medical Device Act" and "AWS Supplier Assessment/Audit Application Reference". Since then, awareness of the security of cloud services has increased, new AWS features have been introduced, and the AWS white paper to read has been updated.
In light of this, we have updated and started providing the two documents with our company, EPS Corporation, Canon IT Solutions, JSOL Corporation, Hitachi Systems, Ltd. and Filler Systems, Ltd.
“AWS Usage Reference for Companies Subject to the Pharmaceutical and Medical Device Act” and “AWS Supplier Assessment/Audit Application Reference”
AWS Usage Reference for Companies Subject to the Pharmaceuticals and Medical Devices Law" covers the concept of organizing requirements and answers in the AWS environment for customers who need to verify their systems based on computerized system management regulations in accordance with the "Guidelines for Proper Management of Computerized Systems" by the Ministry of Health, Labour and Welfare.*The reference includes the concept of organizing requirements in an AWS environment and answers to those requirements. The reference clarifies the scope of responsibility of AWS, the scope of responsibility of system integrators, and the scope of responsibility of customers, and explains the points to be considered when applying CSV.
*Joint Press Release Infographic
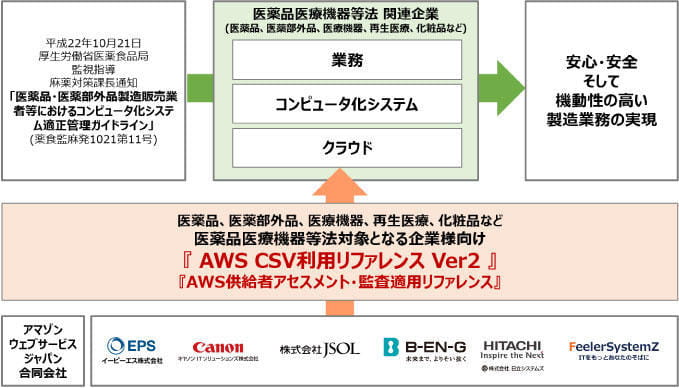
*October 21, 2010 Notice from the Director of the Supervision and Guidance/Narcotics Countermeasures Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labor and Welfare
“Guidelines for Proper Management of Computerized Systems for Pharmaceutical and Quasi-drug Manufacturers and Distributors, etc.” (Pharmaceuticals, Foods and Drugs Administration Bureau No. 1021 No. 11)
Download Document
You can download the following materials.
-
AWS Usage Reference
This material is for companies that are subject to the Pharmaceutical and Medical Device Law, such as pharmaceuticals, medical devices, regenerative medicine, and cosmetics.
Before using this reference, please read the "User License Agreement". You are also deemed to have agreed to the terms and conditions when you begin using the service.- 【Composition】
-
- AWS Usage Reference for Companies Subject to the Pharmaceutical and Medical Device Act Ver.2
(Excel version) - AWS Supplier Assessment/Audit Application Reference Ver.2
(Organizes the points to consider and main points of the supplier checklist in the AWS environment. Explains AWS's proposed answers to the audit items) - Overview of AWS Usage Reference for Companies Subject to the Pharmaceutical and Medical Device Act Ver.2
- License Agreement.pdf *Please be sure to read.
- AWS Usage Reference for Companies Subject to the Pharmaceutical and Medical Device Act Ver.2
Related Solutions
Computerized System Validation (CSV) Support Services
In addition to the system development and implementation team, we have organized a specialized team that provides CSV support services, and it is made up of multiple consultants who have worked in quality assurance at pharmaceutical companies and have many experiences in CSV activities and responding to FDA inspections.

