Manufacturing Execution SystemPAS-X MES for the Pharmaceutical Industry
Werum PAS-X MES (hereinafter referred to as PAS-X) is a manufacturing execution system specialized for the pharmaceutical industry provided by Kerber Pharma, Germany.
PAS-X covers all major lifecycle stages in pharmaceutical and biopharmaceutical manufacturing, from process development to product manufacturing and packaging. We support all major segments of the pharmaceutical manufacturing industry, including vaccines, biopharmaceuticals, solid formulations, and liquid formulations.
As an authorized PAS-X service partner, B-EN-G supports the implementation of PAS-X.
The video below is a message from Oliver, CEO of Körber Pharma Software, to the Japanese pharmaceutical industry.
Overview
Many pharmaceutical manufacturers are facing a dilemma because they are unable to transition from paper forms to electronic forms at the manufacturing site due to concerns about dealing with audit trails and other requirements.
In this context, PAS-X has been developed in compliance with legal requirements such as FDA 21 CFR Part 211 and Part 11, EU GMP and GAMP 5 guidelines, and is therefore recognized by the top 30 pharmaceutical manufacturers in the world. In addition to over half of the companies in Japan, it is also used by many small and medium-sized pharmaceutical manufacturers.
By introducing PAS-X and changing from paper-based to electronic operations, efficiency and compliance will improve.
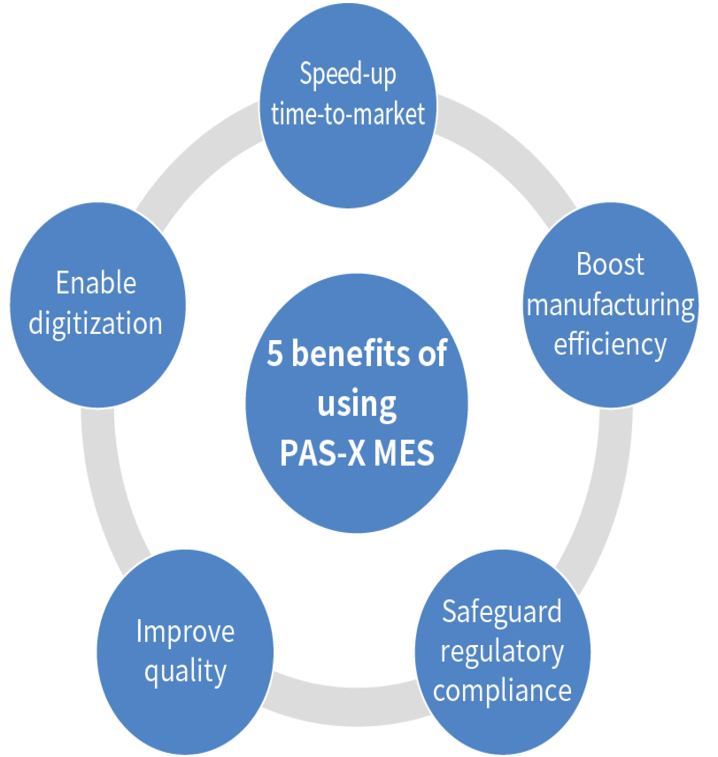
Realized by introducing PAS-X
function
PAS-X implements the unique business requirements of the pharmaceutical and biotechnology industries as standard features, providing a standardized set of functions for these industries and enabling rapid MES start-up.
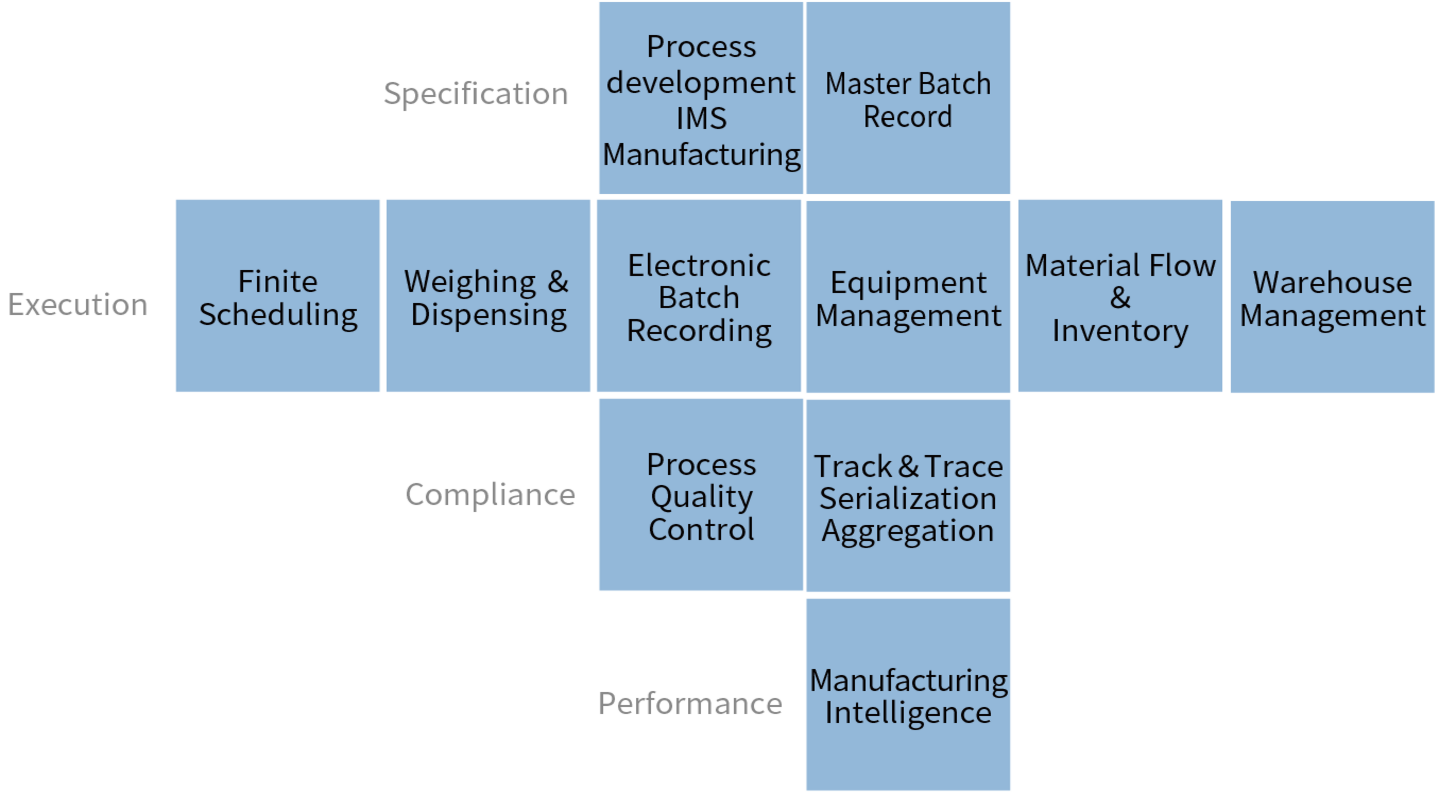
- PAS-X Functions
- Multiple modules available, combine standard features to suit customer needs
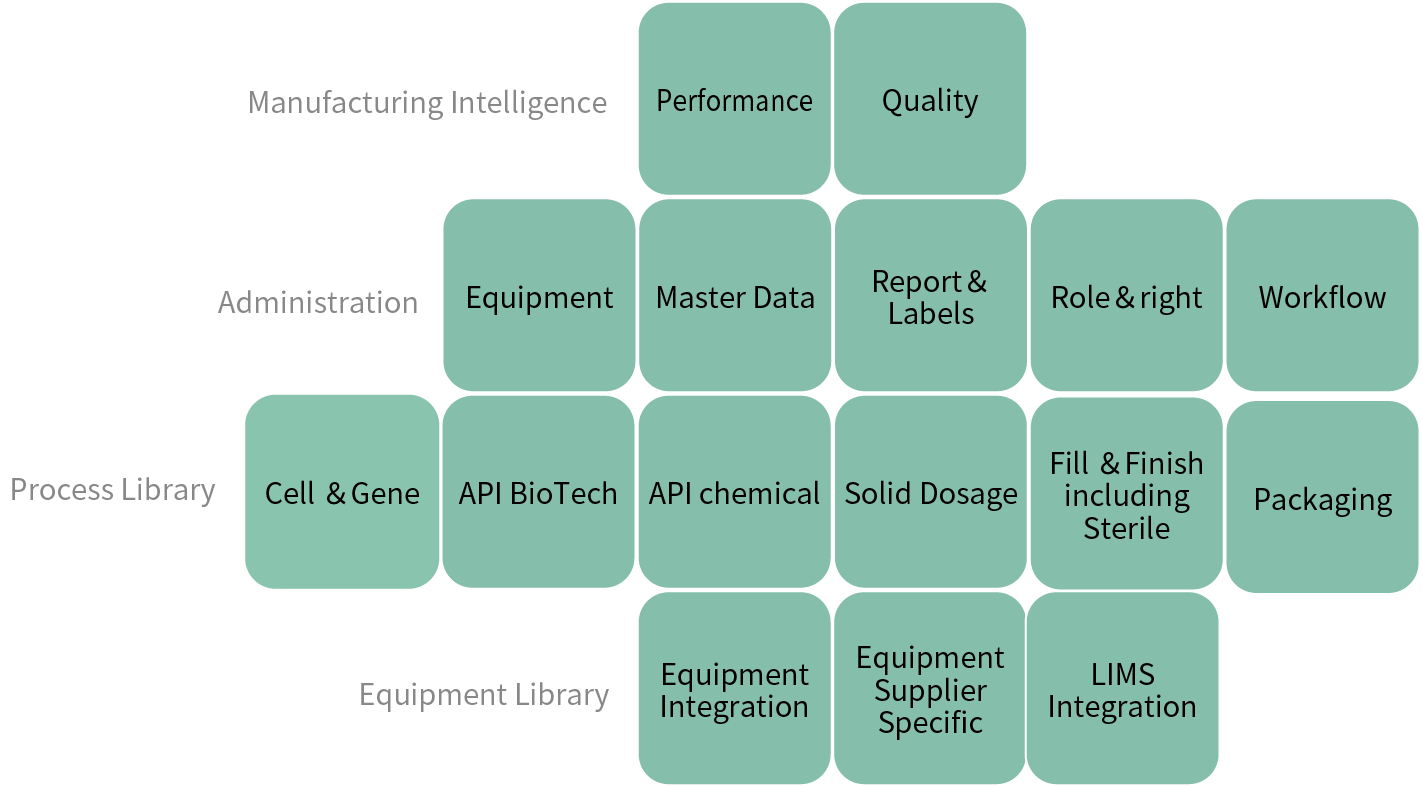
- PAS-X Content
- Save time with industry best practice templates
- PAS-X Service Package
- Accelerating MES Implementation

To achieve Pharma 4.0, B-EN-G will make appropriate implementation proposals, taking into account the customer's unique situation, priorities, and other factors.
| [Example of implementation divided into phases] | |
|---|---|
| Phase 1 | Weighing management |
| Phase 2 | Electronic batch records, master batch records, raw material inventory management |
| Phase 3 | Equipment management, productivity evaluation |
Benefits
- Increase process efficiency
- Improving manufacturing quality
- Monitor and enhance manufacturing capabilities
- Support for compliance with legal and regulatory requirements
- Reduce time to actual production
For a more detailed explanation of PAS-X, please see Kerber's page for pharma.
B-EN-G implementation support
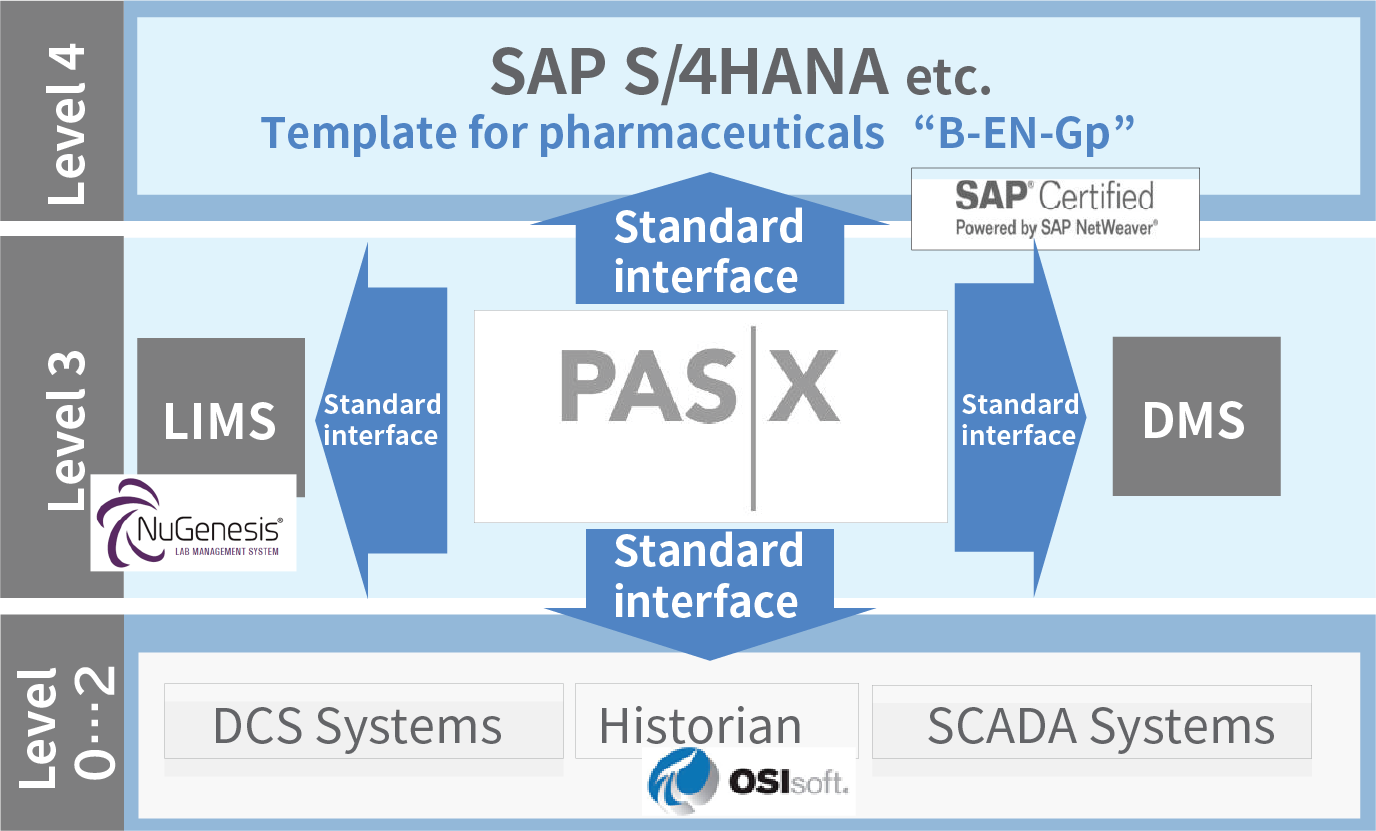
PAS-X uses an interface based on international industrial standards (OPC, XML, etc.), allowing for smooth connections with devices. It is also an integration certified application for SAP ERP.
B-EN-G has over 20 years of experience implementing SAP ERP in the pharmaceutical manufacturing industry and building vertically integrated systems, including the manufacturing execution area. Therefore, we are able to make proposals and implementation that fully utilize the functions of PAS-X MES, which is highly compatible with SAP ERP and SAP S/4HANA. Based on our deep understanding of pharmaceutical production operations, we provide consistent support from concept to system implementation and ongoing support.
Implementation Case Study
Sakamoto Pharmaceutical Co., Ltd., Japan's only specialized glycerin manufacturer, has a new plant at its Senboku factory, which is a core manufacturing site for PAS-X external interface standardization 'Plug and Produce' and a handling solution point for B-EN-G. PAS-X was selected for the "2nd RG facility" which has a production capacity of 20,000 tons. We have strengthened compliance and gone paperless in pharmaceutical manufacturing. During the implementation process, B-EN-G also supported CSV (computer system validation).
For details on the case and to download the leaflet, please see the Sakamoto Pharmaceutical Co., Ltd. PAS-X Implementation Case Study page.
Download Document
- Manufacturing execution system “PAS-X MES” leaflet

Related Solutions
Computerized System Validation (CSV) Support Services
In addition to the system development and implementation team, we have organized a specialized team that provides CSV support services, and it is made up of multiple consultants who have worked in quality assurance at pharmaceutical companies and have had numerous experiences in CSV activities and responding to FDA inspections.
BatchLine Lite MES
Electronic Batch Record (EBR) Solutions for Pharmaceutical and Medical Device Manufacturers

