Electronic Batch Record (EBR) Solution for Pharmaceutical and Medical Device Manufacturers:BatchLine Lite MES
BatchLine Lite MES (hereinafter referred to as BatchLine) is a cloud solution provided by BatchLine, a member of the Factory Group, that enables the electronic recording of manufacturing records for the pharmaceutical (medical device) manufacturing industry.
Since 2004, B-EN-G has been providing services to small and medium-sized pharmaceutical companies in global markets such as Asia, Europe, and the United States as a simple and easy-to-use platform that realizes operational efficiency, strengthens quality control, and optimizes regulatory compliance. B-EN-G has started providing services as a BatchLine implementation partner in the Japanese market since 2024. Pharmaceutical manufacturers that handle a wide variety of formulations can easily proceed with the electronicization of manufacturing records.
This video introduces BatchLine LiteMES, which enables electronic production records for the pharmaceutical (medical device) manufacturing industry.
Overview
BatchLine is a SaaS electronic batch record (EBR) solution.
It reduces the time and effort required to create, review and approve batch records and its rapid deployment and validation methodology ensures smooth implementation while meeting GMP/FDA regulatory compliance requirements, catering to the requirements and budgets of medium to large scale pharmaceutical manufacturers.
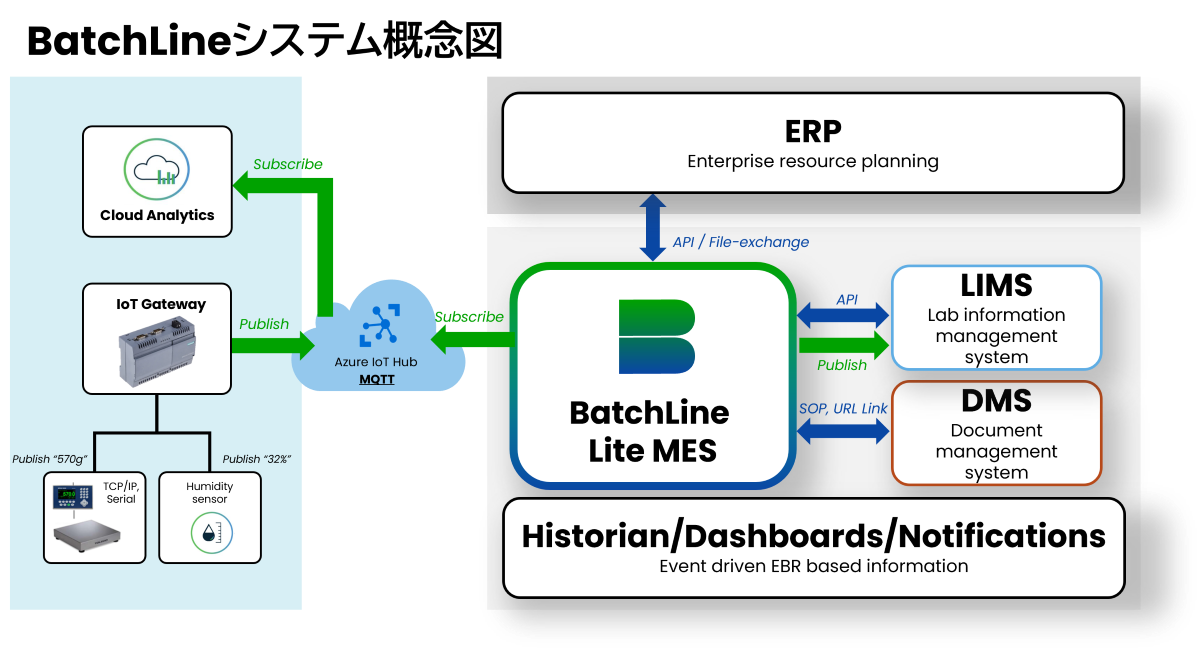
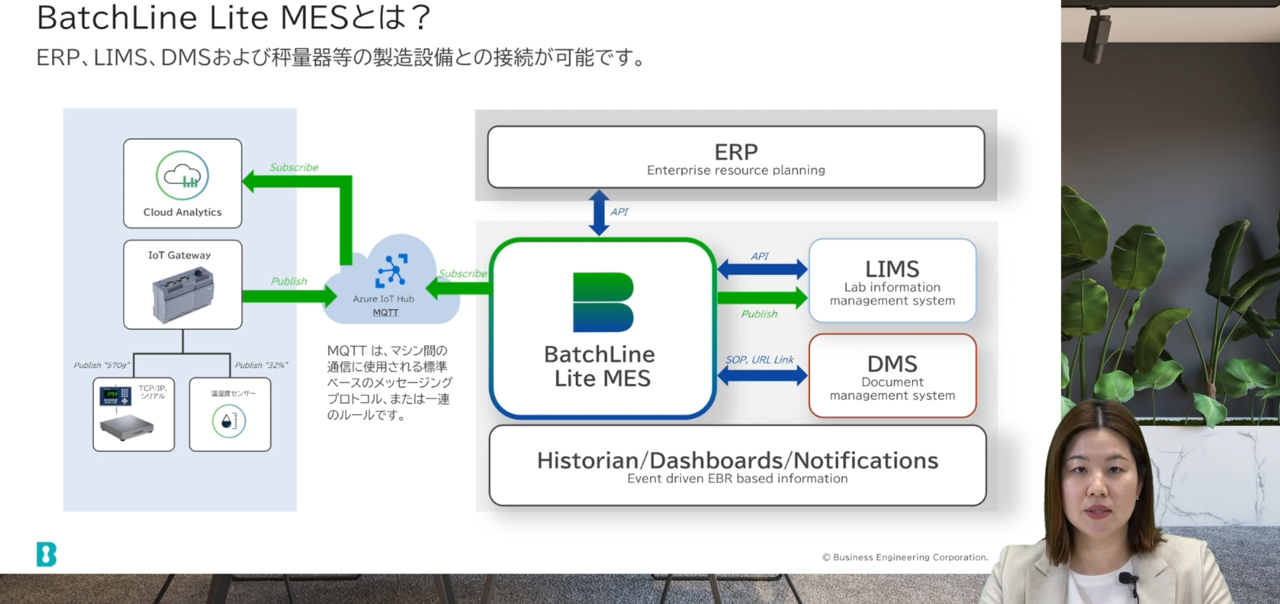
function
BatchLine digitizes your production records.
- Designed to comply with CFR Part 11, Annex 11, PIC/S GMP, EU and US FDA data integrity guidelines
- Real-time reporting and analytics dashboard for factory-wide progress and compliance
- Review by Exception for Faster Batch Release
- Deviation management and audit trail support
- Can be connected to ERP, LIMS, DMS, and manufacturing equipment such as weighing scales
Features
BatchLine is a solution that makes it easy for anyone to achieve digitalization in the pharmaceutical and biotechnology industries in compliance with GxP standards.
The master settings before creating the MBR are simple, so users can easily create and operate MBRs based on them themselves.
Benefits
By digitizing paper production records, it becomes possible to visualize the factory status in real time.
■Improvement of quality control and business efficiency
- Automatic generation of final batch report
- Reduce time spent on exception reviews and QA processes
- Check data entry against specifications as it is entered
- Shortening time from production to batch release
■ Cost reduction
- Reduce operational costs while remaining compliant
- Significant reduction in paper-related operational costs (paper costs, printing, filing, storage costs, etc.)
- Improved efficiency in searching for deviations, etc., significantly reduced the amount of work required for QA
B-EN-G implementation support
The pharmaceutical manufacturing execution system (MES) is a system that instructs workers to manufacture in accordance with the contents of the approval documents and records the results of the work. The results of the work are recorded along with the work content, when and who performed it, and if the recorded content is rewritten, it is also recorded who rewrote it and why.
B-EN-G offers two products, "PAS-X" and "BatchLine Lite MES," as MES solutions specialized for pharmaceutical manufacturing.
We propose and support the implementation of MES-related solutions tailored to the challenges faced by each customer.
Related videos
- A consultant will give an overview of "BatchLine Lite MES," an electronic batch record (EBR) solution for pharmaceutical and medical device manufacturers. (8 minutes)
Related Solutions
Computerized System Validation (CSV) Support Services
In addition to the system development and implementation team, we have organized a specialized team that provides CSV support services, and it is made up of multiple consultants who have worked in quality assurance at pharmaceutical companies and have had numerous experiences in CSV activities and responding to FDA inspections.
PAS-X Manufacturing Execution System for the Pharmaceutical Industry
A manufacturing execution system that covers all major lifecycle stages of pharmaceutical and biopharmaceutical manufacturing, from process development to product manufacturing and packaging.

