Challenges
- With the opening of a new plant, it was necessary to introduce a manufacturing execution system that meets pharmaceutical quality requirements.
- It was essential to streamline the process of creating manufacturing records and go paperless in compliance with GMP (Good Manufacturing Practice) for pharmaceutical manufacturing.
- The MES introduced to achieve complete automation required automatic coordination with various manufacturing equipment such as ERP and DCS (Distributed Control System).
Implemented Products/Solutions
Manufacturing Execution System for the Pharmaceutical Industry “Werum PAS-X MES”
Project Background
Sakamoto Yakuhin Kogyo's core business is the development, manufacture, and sale of 100% plant-derived glycerin and its derivatives, based on the vision of "Creating the future from glycerin." The Senboku Plant in Osaka serves as the company's core glycerin manufacturing base. The plant has acquired ISO 9001 and ISO 14001 certification, as well as licenses to manufacture pharmaceuticals and food additives, and the RSPO (Roundtable on Sustainable Palm Oil) supply chain certification system MB (Mass Balance) certification, which guarantees sustainable palm oil production, and other certifications, and is able to stably manufacture and supply high-quality glycerin products. In February 2022, a new plant, the No. 2 RG facility, with an annual production capacity of approximately 20,000 tons, began operation.
“What the Senboku Factory is pursuing is not only to stably produce high-quality glycerin products with excellent environmental performance, but also to increase production efficiency by making full use of digital technology and data, and ultimately to improve manufacturing efficiency. Our goal is to achieve full automation. The new plant we built with this purpose in mind is the 2nd RG facility,'' explains Yoshihiro Mine, manager of the Senboku Plant.
One of the systems adopted in the 2nd RG facility to improve production efficiency is the "Werum PAS-" Manufacturing Execution System (MES) specialized for the pharmaceutical industry, for which B-EN-G provides implementation support services. X MES (hereinafter referred to as “PAS-X”).
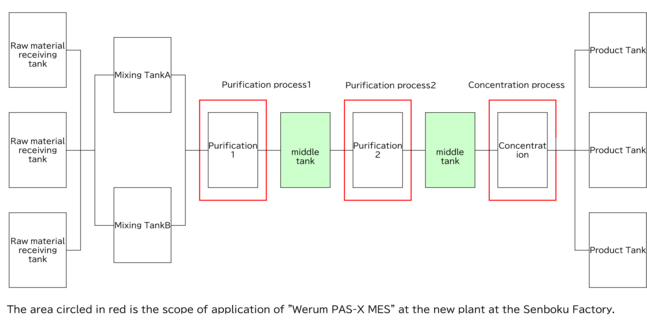
Implementation Results
- A GMP-compliant plant was completed by utilizing PAS-X, a package specialized for the pharmaceutical industry.
- Achieved automatic collection of manufacturing performance data from manufacturing equipment and paperless manufacturing records.
- Achieving automatic linkage between ERP and various manufacturing equipment and PAS-X, creating an environment where manufacturing can be started with touch operation on a tablet, and realizing manufacturing automation.
- Use of reliable MES also contributes to the audit response of the pharmaceutical company they supply to


Implementation Points
- A project management officer was appointed within the project and a system was established to coordinate multiple participating vendors.
- Adoption of PAS-X, which has a rich track record of implementation in the pharmaceutical industry
- Support from B-EN-G, which has extensive knowledge and experience in pharmaceutical manufacturing and computerized system validation (CSV).
Case study company introduction
| Company Name | Sakamoto Pharmaceutical Co., Ltd. Sakamomto Yakuhin Kogyo Co., Ltd. |
| Establishment | May 31, 1951 |
| Head office location | Chuo Ward, Osaka City, Osaka Prefecture |
| Business Activities | Manufacture and sale of glycerin, polyglycerol fatty acid esters, cosmetic raw materials, flame retardants, etc. Sale of organic and inorganic chemicals, etc. |
| Business Locations | Head office, branch office, research laboratory, three domestic production sites (Senboku Factory, Ako Factory, Daito Factory), Philippine production site (Sakamoto Orient Chemicals Corporation) |
| Company website | https://www.sy-kogyo.co.jp/ |
*Please note that organization names, positions, numerical data, etc. in the article are based on the time of the interview and may have changed by current viewing.
Related Solutions
Relevant information and case studies based on solutions presented above.
Related Case Studies

Nxera Pharma Japan Co., Ltd. (Formerly Idorsia Pharmaceuticals Japan Ltd.)
Compatible with Global Requirements: Case Study of a Project to Build a Sales Management System Compatible with JD-NET
Medicine
ERP
Pharmaceutical & Medical Device Industries
SAP

GE Healthecare Pharma Limited
Promoting Understanding among Global IT Teams: JD-NET-compliant Enterprise Resource Planning System Implementation Project Case Study
Medicine
ERP
Pharmaceutical & Medical Device Industries
SAP

KAKEN PHARMACEUTICAL CO., LTD.
Case Study of Introducing a New Accounting System for Regulatory Compliance and Company-wide Business Optimization
Medicine
ERP
Pharmaceutical & Medical Device Industries
SAP

