Ethics/Volunteer System
Tests using human-derived samples and information during the drug discovery process must be conducted in accordance with the "Ethical Guidelines for Medical Research Involving Human Subjects" established by the government.
The "Ethics/Volunteer System" (bReev-CS) flexibly responds to these ethical guidelines and realizes smooth execution of research ethics review, research implementation, and management without violating compliance.
Overview
We provide services in the cloud, covering everything from ethics applications to volunteer recruitment and implementation of samples needed for research.
Since we use Microsoft Azure as our platform, we have thorough security measures such as communication encryption and data encryption. In addition, data security such as data backup is fully ensured.
機能
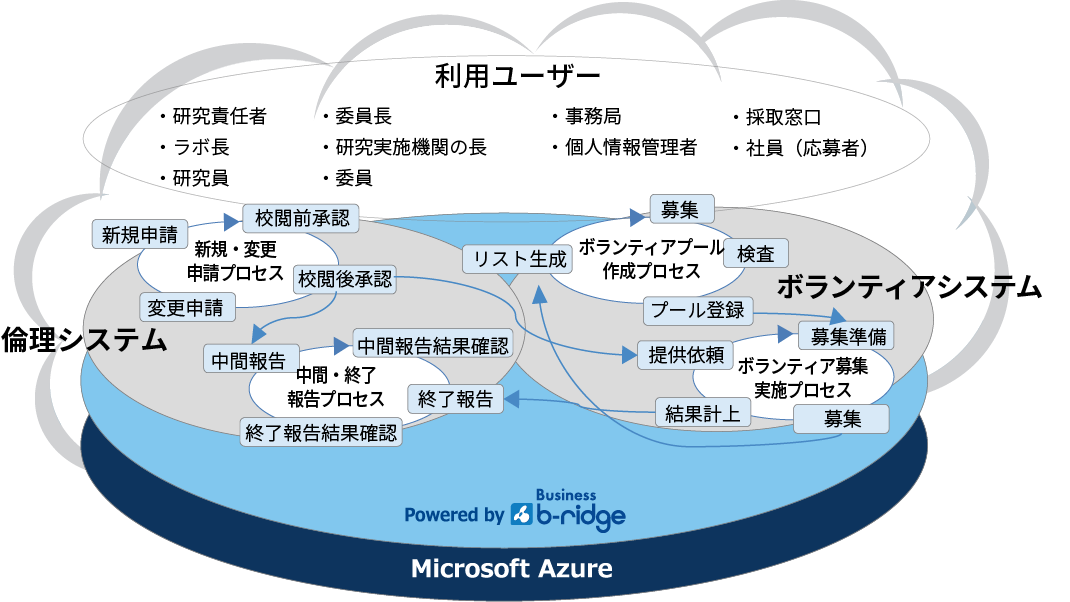
As shown in the diagram, it consists of two systems: an ethics system and a volunteer system.
導入効果
- Streamline complex operations
- A checklist of compliance items for the application form is prepared and provided as a template at the time of application to accommodate various patterns. When revisions are made, the template is revised to quickly respond to the latest format.
- Prevent communication loss
- Equipped with a message function that fills in the lines of the ledger to improve screening speed and facilitate inter-process collaboration. Emails are sent to the appropriate people when necessary. Also allows grouping of recipients to prevent misdirects.
- Compliance
- By changing the standard settings, it is possible to operate the system without violating compliance by defining the "reference range" for each role at the management item level, such as hiding the personal information protection part from all but specific roles.
Implementation Case Study
Download Document
You can download the following materials.
- Ethics/Volunteer System Leaflet
Describes service overview, implementation effects, and function overview