Cloud Qualification Service
For companies subject to the Pharmaceuticals and Medical Devices Act, including pharmaceuticals, medical devices, regenerative medicine, and cosmetics, the Japanese Ministry of Health, Labour and Welfare requires that Computerized Systems Validation (CSV) be performed when computerizing operations under legal regulations (GxP operations).*.
B-EN-G provides qualification services as CSV for infrastructure when cloud services are adopted.
*October 21, 2010 Notice from the Director of the Supervision and Guidance/Narcotics Countermeasures Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labor and Welfare
“Guidelines for Proper Management of Computerized Systems for Pharmaceutical and Quasi-drug Manufacturers and Distributors, etc.” (Pharmaceuticals, Foods and Drugs Administration Bureau No. 1021 No. 11)
What is the concept of CSV in cloud utilization?
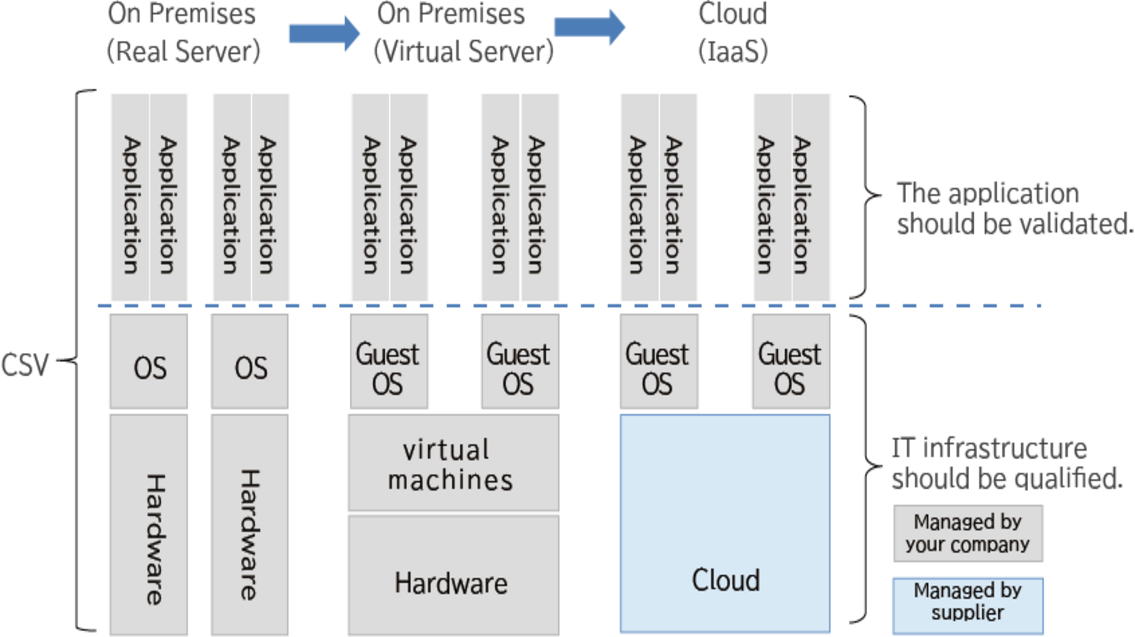
As IT technology has advanced and server virtualization technology has emerged, CSV for physical servers has become an inconvenience because it requires multiple checks of the physical servers if CSV is conducted as an integrated part of the server. The concept of separation of application and infrastructure was proposed in EU GMP Annex 11, which was revised in 2011, and has been globally adopted in PIC/S GMP Annex 11, which was revised in 2013.
As a result, the traditional CSV methodology can be utilized for applications, and for infrastructure qualification, the methodology can be tailored to the cloud services used.
B-EN-G Qualification Service
When using cloud services, it is also important to identify risks that cannot be directly managed, conduct a risk assessment, understand whether there is a risk or not, and think in advance how to respond in the event of a risk.
Microsoft Japan, which provides Microsoft Azure (hereinafter referred to as Azure), has obtained many third-party certifications such as ISO9001 and discloses this information on its website. We also publish technical information as a white paper, so by evaluating this published information and compiling it into a supplier evaluation report, it can be used in place of the IQ and OQ of the cloud service part.
At B-EN-G, we are aware of the difference between on-premises and cloud qualification, and we provide services ranging from infrastructure qualification planning when using Azure to risk assessment, supplier evaluation, and completion of IQ/OQ plans/reports.
Microsoft Azure Qualification Practical Training for CSV Personnel
This is a training service for CSV professionals who understand CSV but do not know how to perform Azure qualification for infrastructure configuration.
The one-day course includes lectures in the morning, exercises in the afternoon, and is held in small groups.
Consultants with extensive practical experience serve as instructors.
- Advantages of taking the course
- CSV staff will be able to carry out Azure qualification themselves using the templates of deliverables (Azure Qualification Kit) packed with Azure qualification know-how and the templates used in the exercises.
- Training goals
-
- Gain ability to explain the concept of qualification in cloud environments
- Learn how to create a qualification plan using the provided templates
- Learn how to conduct risk assessments and supplier evaluations using the provided templates
Download Document
You can download the following materials.
-
White Paper “Microsoft Azure Qualification”
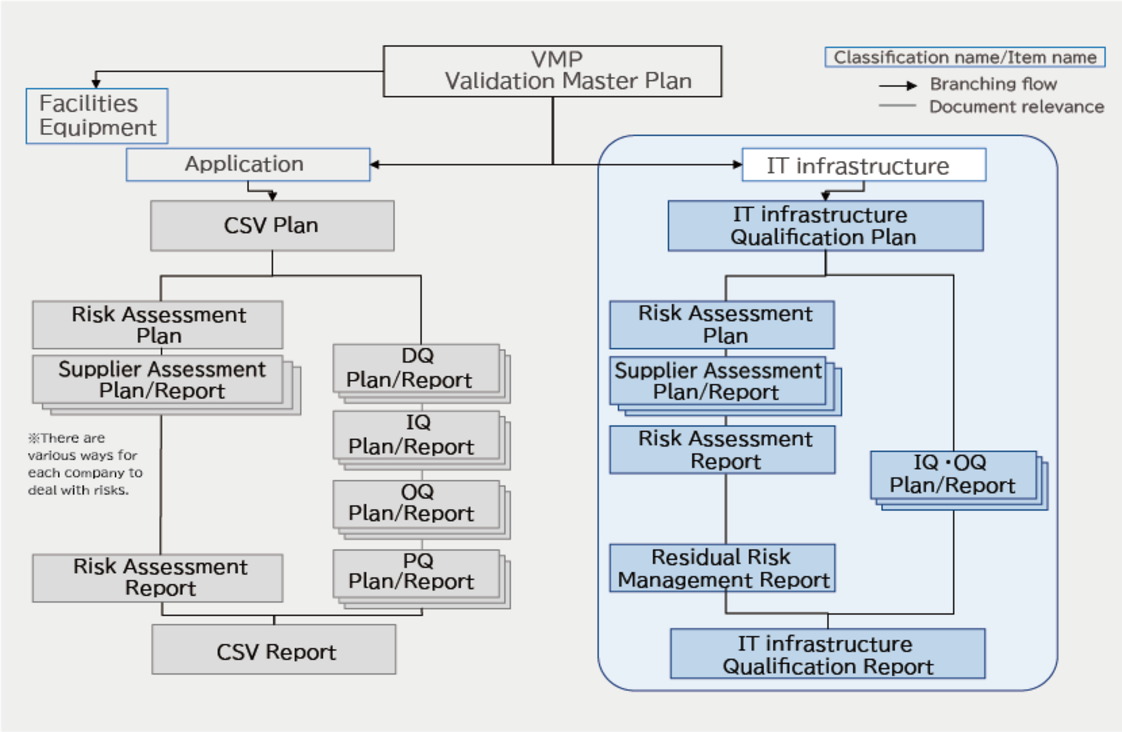
This is a detailed explanation of the contents of this page and an introduction to the "Azure Qualification Kit," which is a template for a set of qualification documents using B-EN-G's Azure. This document is intended for companies subject to the Pharmaceuticals and Medical Devices Act, including pharmaceuticals, medical devices, regenerative medicine, and cosmetics.
- [Main Content]
-
- Changes in CSV method
- Azure qualification
- CSV document system
- Azure Qualification Kit contents

Related Solutions
- Computerized system validation support services
In addition to the system development and implementation team, we have organized a specialized team that provides CSV support services, and it is made up of multiple consultants who have worked in quality assurance at pharmaceutical companies and have many experiences in CSV activities and responding to FDA inspections. - Microsoft Azure
Please check the Microsoft Japan website for details.

